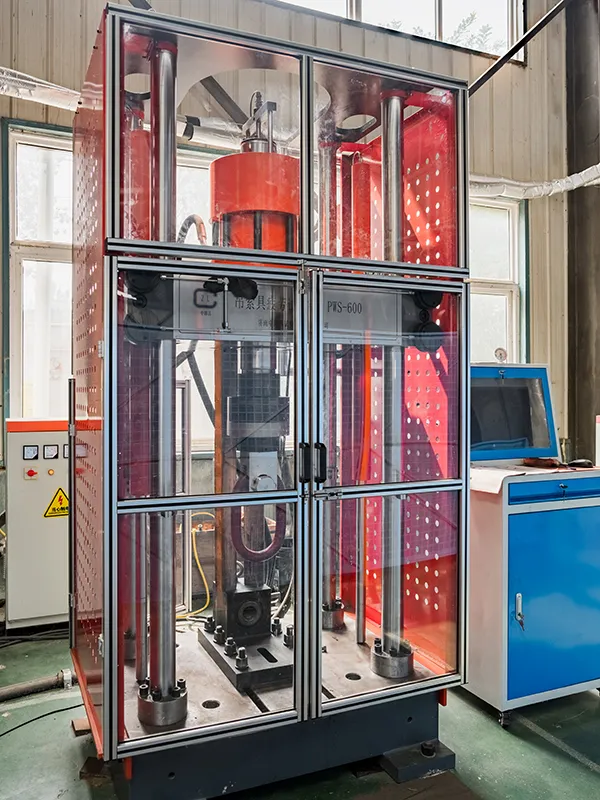
Quality control is essential to monitor and eliminate factors causing non-conformance or unsatisfactory results at every stage of production, ensuring compliance with quality requirements and achieving economic efficiency. At Mainlywell, we operate a fully equipped materials analysis laboratory and utilize advanced quality testing equipment, including digital Rockwell hardness tester, metallographic analysis instrument, fatigue tester, 1000KN-2000KN tensile testing machines, and salt spray tester. Our rigorous inspections span all stages, from raw material procurement to production and final product release, with detailed records maintained for every inspection phase. All lifting equipment and material handling products undergo comprehensive testing and certification before leaving the factory. We are committed to providing you with verified and reliable lifting solutions.

Testing and Measuring of Raw Materials
- Appearance: Visual inspection and comparison against reference samples
- Dimensions: Verified using calipers, micrometers, and other measuring tools
- Properties: Mechanical characteristics tested with specialized instruments and methods

Quality Control During Production Process
- Self-inspection and cross-inspection conducted on the first product of each day
- 100% inspection of key dimensions
- Inspections performed at each stage of the production process
- Final inspection upon product completion
- Combination of sampling inspections and full inspections

Inspection After Heat Treatment
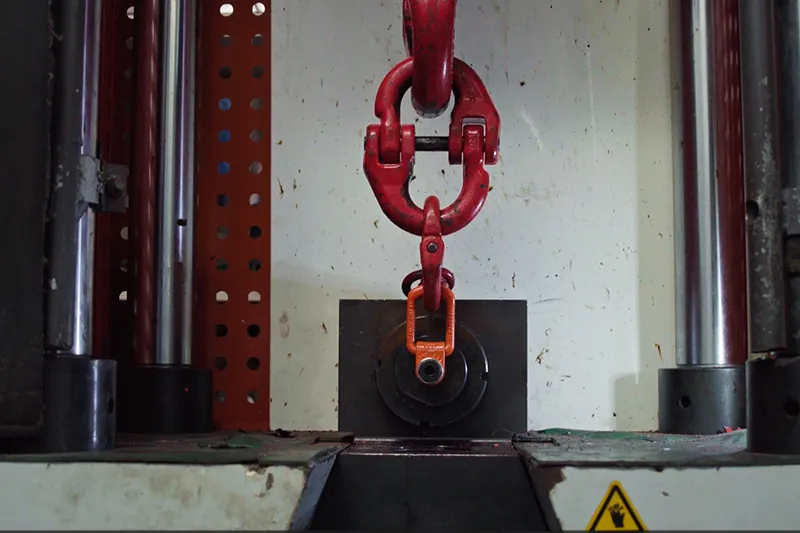
- 100% static load testing of lifting points at 2.5 times their rated capacity
- Hardness testing conducted via sampling and cross-inspection
- Magnetic particle flaw detection performed through sampling and cross-inspection

Final Inspection of Finished Products
- Finished products inspected before shipment
- 100% appearance inspection
- 100% thread inspection using pass/fail gauges
Unqualified Products Feedback and Treatment
- Defective items identified and addressed immediately
- Suspected defective parts submitted for supervisor review, followed by corrective action or treatment
- Records of abnormalities maintained
- Corrective and improvement measures verified and tracked
- Defective semi-finished or finished products effectively separated
Quality Record
- Records must be accurate, timely, clear, and complete, with inspection stamps or signatures.
- Records are promptly organized, archived, and stored in a suitable environment.